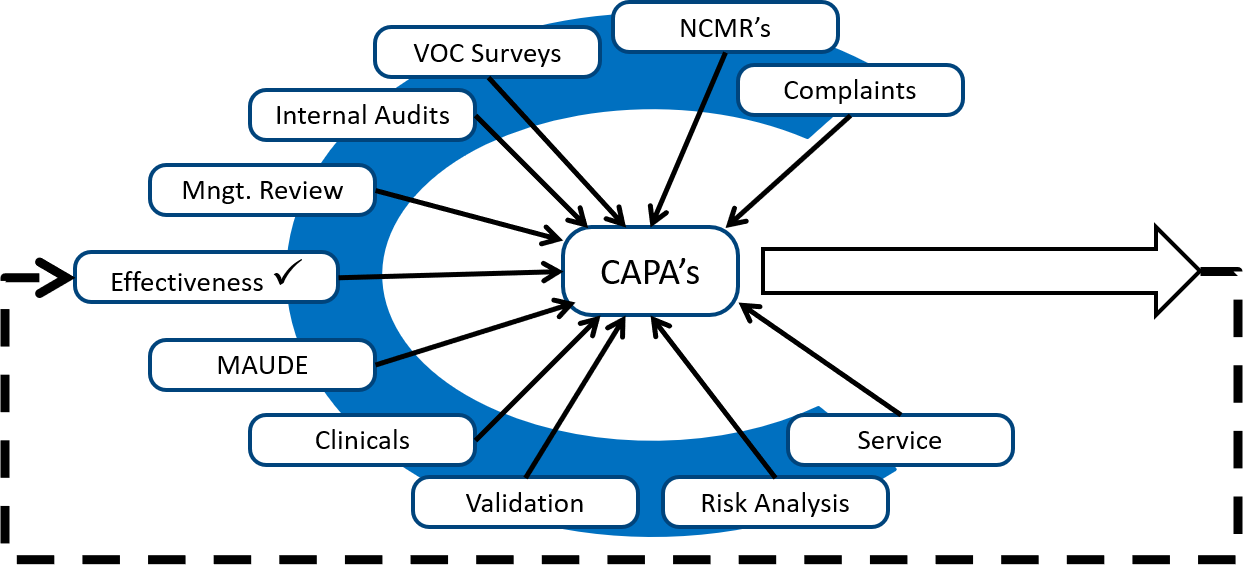
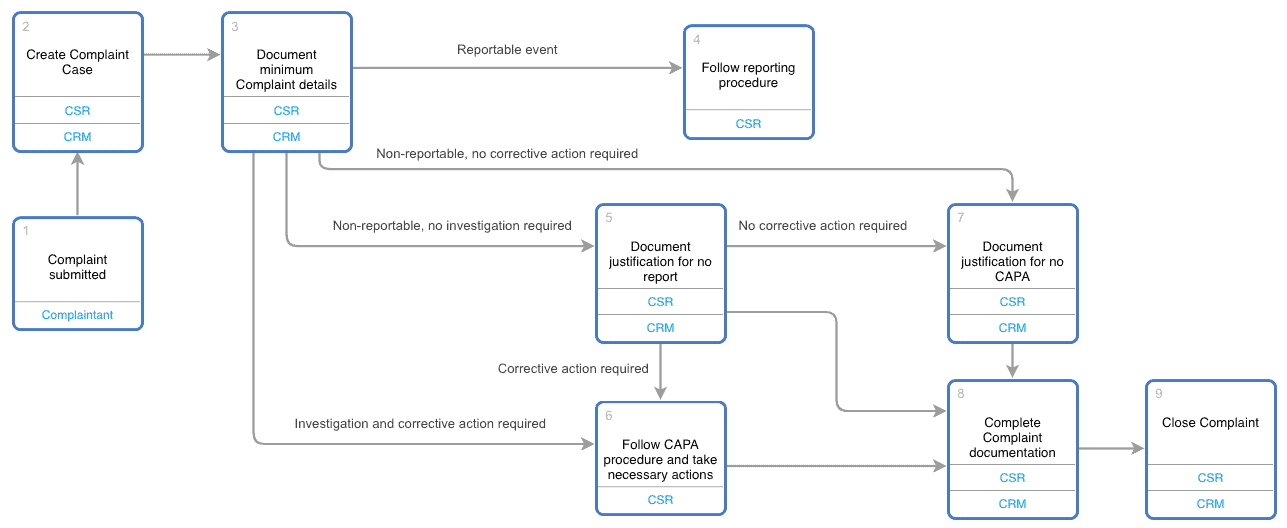
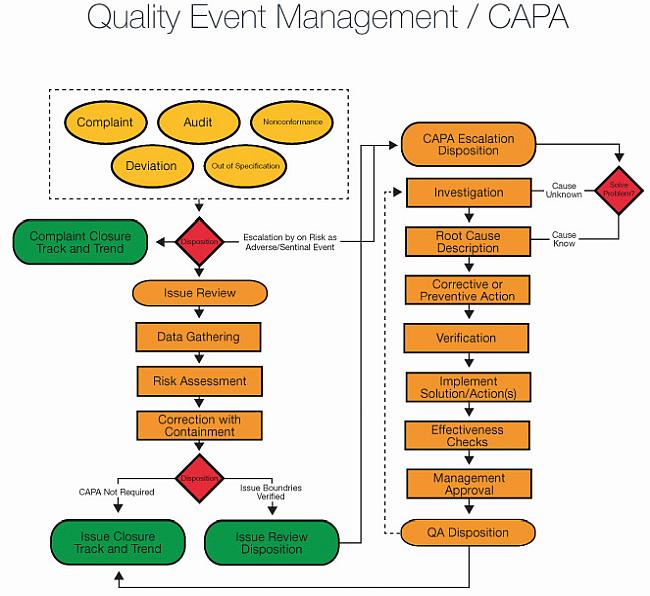
Complaint Handling Requirements - Interrelationship with CAPA, Change Control, Adverse Event Reporting and Recalls, Life Cycle Process Activities - Webinar Compliance

PDF) Corrective and Preventive Action (CAPA) and Complaint Handling in Medical Device. - By Compliance Global Inc | Compliance Global Inc - Academia.edu