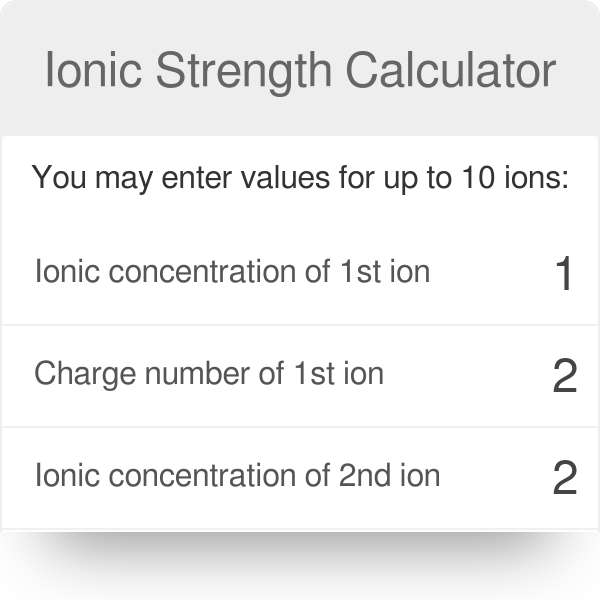
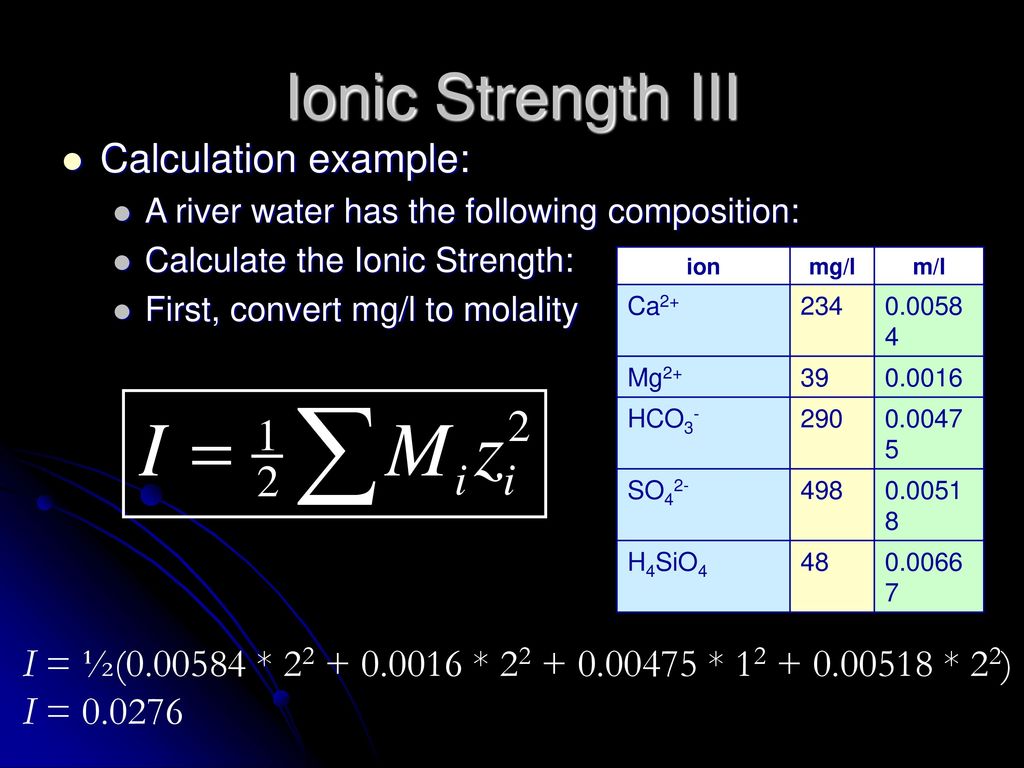
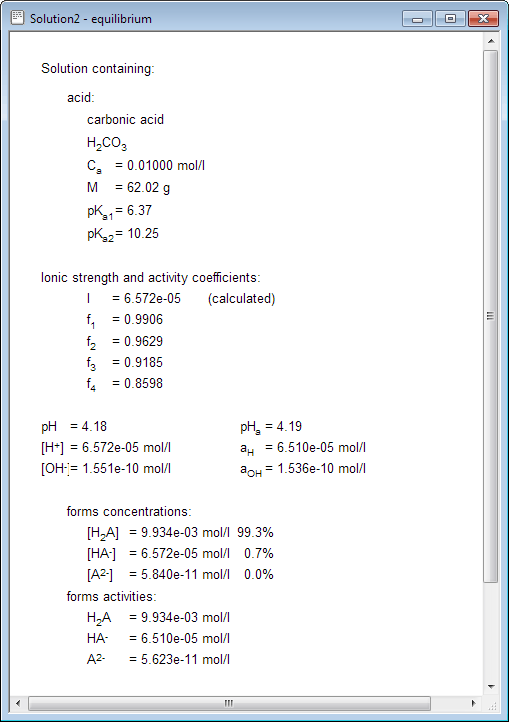
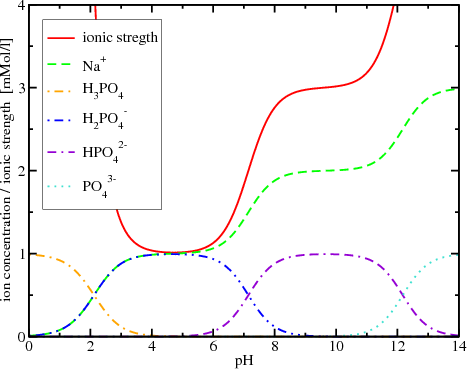
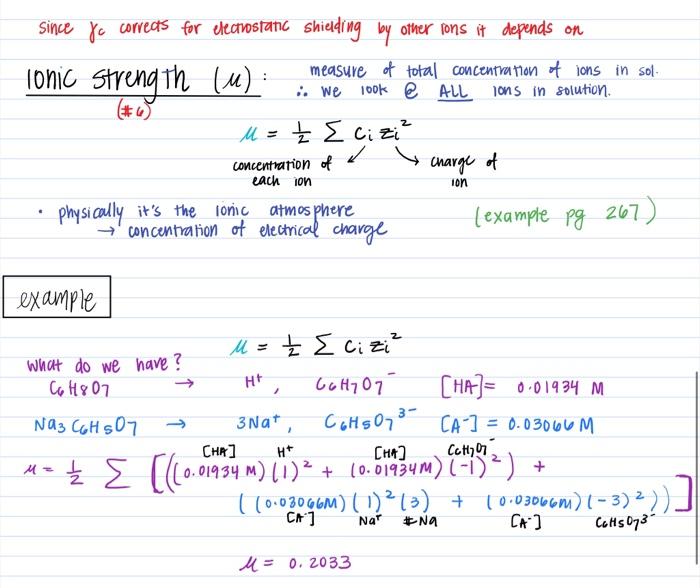
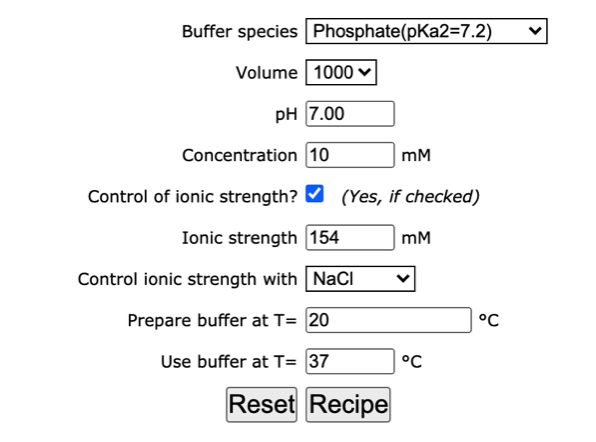
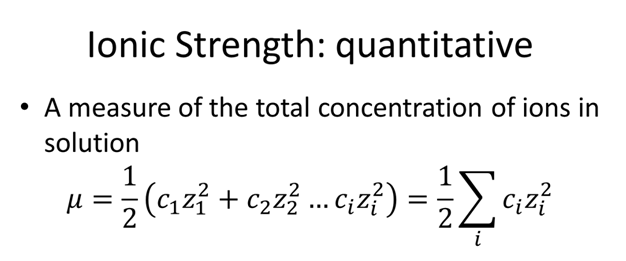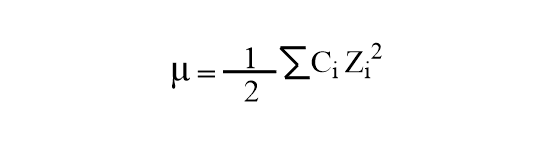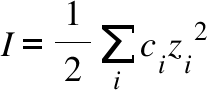pH calculations and more in fundamentals of pharmaceutics. : What is ionic strength of solutions and how is it calculated?

Error: Ionic Strength out of Range - The Geochemist's Workbench - Geochemist's Workbench Support Forum

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of